Odor neutralization technology
Odor neutralization is achieved through the interaction of active agents that, upon contact with malodorous molecules, effectively reduce olfactory discomfort.
Depending on the medium in which the source of the odor is found, nebulization, micronization, spraying, or evaporation technology will be used.
Once the active neutralizing agents come into contact with the malodorous molecules, mechanisms of action occur that convert them into non-volatile or odorless compounds.
How it works
Diagram of how Biopulcher products work in odor elimination
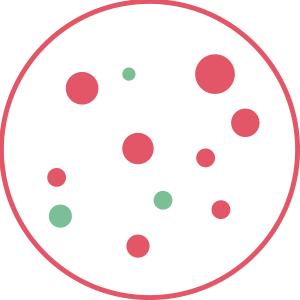
1. Bad odors
The bad odor (red circles) is caused by particles present in the air.
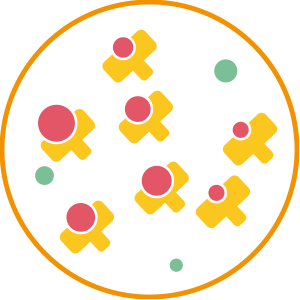
2. Application
After application, the Biopulcher odor removers products captures the molecules responsible for the bad odors to bind with them.
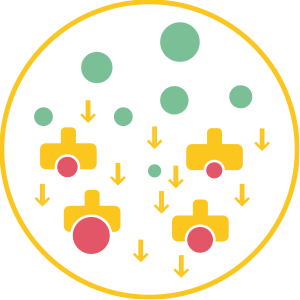
3. Precipitation
This causes an increase in the weight of these particles and their precipitation, removing them from the air.
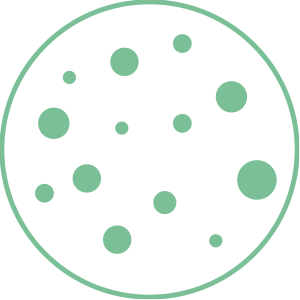
4. Clean air
The air is instantly free from particles that cause bad odors.
The main mechanisms of action are:

Absorption
Absorption
Odorous volatile compounds adhere to the surface of our active agents, resulting in non-volatile, odorless clusters.

Chemical reaction
Active principles chemically react with malodorous molecules, transforming them into odorless or less volatile molecules.
Odor neutralization technologies
The most common chemical reaction mechanism of our neutralizers involves a nucleophilic attack on the nitrogen and sulfur atoms of the most frequent odor molecules such as amines, sulfides, or thiols.
These molecules are commonly found in odors from garbage, fish, sewage, urine, and feces. Odor neutralizers initiate chemical reactions with the carbonyl group of aldehydes, ketones, and some esters, such as the following:
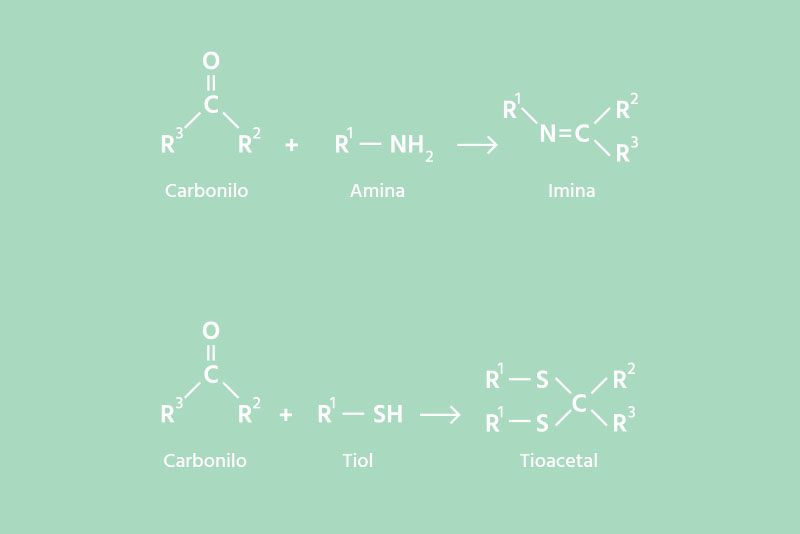
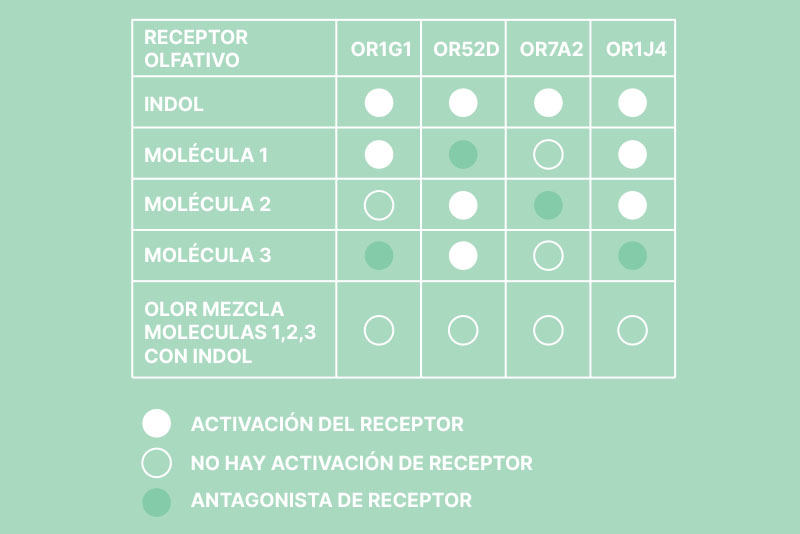
When dealing with malodorous molecules with very low perception thresholds, such as indole (1.4 micrograms/m3 of air) or even lower, such as methylamine (0.021 micrograms/m3 of air), neutralizers are often insufficient to reduce their concentration below their perception thresholds. In these cases, we use specific antagonists of the olfactory receptors for these molecules, which produce temporary anosmia, only for that specific odor.
For example, in the case of indole, present in human and animal feces: Indole primarily activates olfactory receptors OR1G1, OR52D, OR7A2, and OR1J4. This combination is responsible for its odor. Molecule 1 antagonizes receptor OR7A2; molecule 2 antagonizes receptor OR1G1, and molecule 3 antagonizes receptor OR1J4. When we mix indole molecules with the three molecules from the table in the environment, the odor of indole is no longer perceived.
What sets us apart
At Biopulcher, we are committed to innovation, continuously developing new formulas and technologies to address olfactory issues
- We work closely with our clients to understand their specific needs and provide customized solutions.
- Unlike other companies, we are not distributors; rather, we are manufacturers committed to the development and formulation of our own products.
- With a robust R&D department, we can offer tailored solutions that perfectly fit each client's needs.
- Furthermore, by eliminating intermediaries, we can offer more competitive prices, delivering greater value to our customers.
Live with pets, not with their smell !
Bioenzymatic, vegan and 100% biodegradable odor eliminator
Powerful bioenzymatic, vegan and biodegradable odor eliminator that quickly and effectively removes pet-related smells such as body odor, urine, feces, vomit, saliva, and more.
Works instantly to keep areas where your pets rest such as sofas, beds, fabrics and carpets smelling fresh while also neutralizing stubborn odors in litter boxes.
It also helps prevent urination on floors and walls.











